Unit 12. Hydrocarbons (FBISE SSC-II Chemistry Keybook)
2. Give short answers.
i. Give three examples of unsaturated hydrocarbons?
Hydrocarbons containing carbon-carbon multiple bonds are called unsaturated.
|
Ethene |
1-Pentene |
1-Butyne |
|
|
|
|
- Draw electron dot and cross structure for ethene.
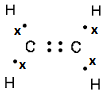
- Draw structural formula of an alkane, an alkene and an alkyne containing five carbon atoms.
|
Alkane (Pentane) |
Alkene (Pentene) |
Alkyne (Pentyne) |
|
|
|
|
- How can you differentiate between ethane from ethene?
- Add a small amount of bromine water to each jar.
- Shake the jar containing ethane will de-colour the bromine water.
- The jar containing the ethane the bromine water will remain brown.
- This is because the Br2 will add on across the double bond of the unsaturated ethane to produce dibromoethane.
- The Br2 is removed from the water which becomes clear.
- In the case of ethane this is already saturated, so no reaction occurs – Bromine water remains brown.
- What do you mean by dehydration reaction? Give one example
Dehydration means a chemical reaction that involves the loss of a water molecule from the reacting molecule.
Example:
Alcohols dehydrate when their vapour are passed over heated alumina.

3. How can you convert?
i. Ethene into ethane:

diffused
ii. Methane into Carbon tetrachloride:
CH4 + Cl2 CH3Cl + HCl
CH3 – Cl + Cl2 CH2Cl2 + HCl
CH2 – Cl2 + Cl2 CHCl3 + HCl
CHCl3 + Cl2 CCl4 + HCl
Sunlight
Sunlight
Sunlight
Sunlight
diffused
diffused
diffused
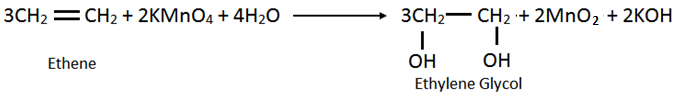
iii. Ethene into glycol:
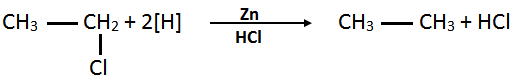
- Ethyl Chloride into ethane:
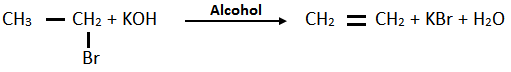
v. Ethyl bromide into ethene:
4. Write a chemical equation to show the preparation of an alkane from an alkene and an alkyne.
![]()
![]()
5. Write a chemical equation to show the preparation of ethane from dehydration of an alcohol and dehydrohalogenation of alkyl halides.
a. By Dehydration of Alcohols:

b. By dehydrohalogenation of alkyl halides:
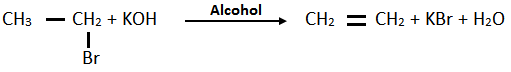
6. Write a chemical equation to show the preparation of ethyne from dehalogenation of 1, 2 – dihalide and a tetrahalide.
a. By dehydrohalogenations of 1,2 – dihalide:

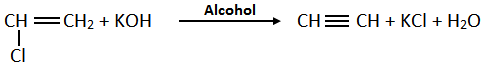
b. By Dehalogenation of Tetrahalides:
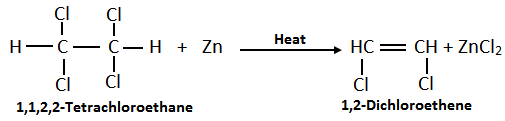

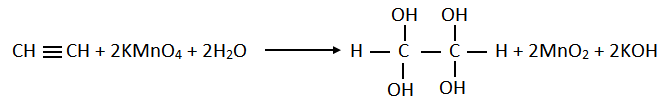
7. Write chemical equation showing reactions of KMnO4 with ethene and ethyne.
a. Reaction with Ethene:

b. Reaction with Ethyne:
8. List some industrial uses of ethene and ethyne.
Uses of Ethene:
- Ethene is used for artifical ripening of fruits.
- It is used in the manufacture of plastics, such as packing films, wire coatings, and squeeze bottles.
- It is used as antifreeze in automobile radiators.
Uses of Ethyne:
- In oxy-acetylene torch for welding and cutting metals.
- For ripening of fruits.
- For the manufacture of polyvinyl acetate (PVA), polyvinyl chloride (PVC), polyvinyl ethers and rubbers.
9. Explain why a systematic method of naming chemical compounds is necessary.
There are millions of organic compounds. It is impossible to study individual compound. To understand, recognize and classify these compounds, systematic naming of organic compounds is necessary. Organic chemists devise a system naming organic compounds depend on their structure. An international body, the International Union of Pure and Applied chemistry (IUPAC) constantly reviews the rules for naming organic compounds. IUPAC system of naming organic compounds is based on the following principle.
“Each different organic compound should have a different name.”
10. Draw electron dot and cross structure for:
|
Propane |
Propyne |
Propene |
|
|
|
|
11. Write chemical equations for the preparation of propene from:
CH3 – CH2 – CH2 – OH CH3–CH = CH2 + H2O
170oC
CH3 – C = CH + 2H2 CH3 – CH = CH2
200 – 300oC
Ni
12. Write down structural formulas for the products which are formed when 1-butene is reacted with:
- H2 / Ni
![]()
- Dilute alkaline aqueous KMnO4 solution

- Bromine water

- Chlorine

13. Identify A, B, C and D in the following reactions.

Solution:
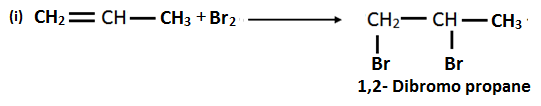
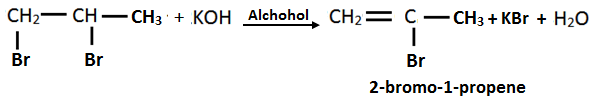
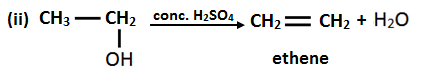

14. How can you convert ethene into ethane?
Ethene is converted into ethane by the process of hydrogenation. Hydrogenation takes place in presence of finally divided nickel at 200 – 3000C and high pressure.
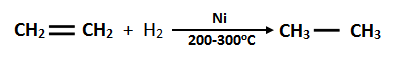
15. You are given two flammable liquid hydrocarbons. One of them is an alkene and other is an alkane. How would you find out which is which?
Bromine water is a dilute solution of bromine, which is a brownish red liquid. This becomes a colourless solution when mixed with an alkene or unsaturated fats.

But when mixed with alkanes or saturated fats, the colour remains the same.
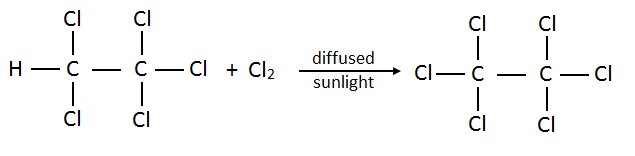
16. How many possible products are there when chlorine reacts with ethane? Draw them all.
The reaction of ethane and chlorine in diffused sunlight occurs as follows.
- Chloroethane:
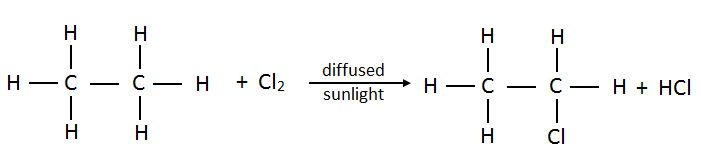
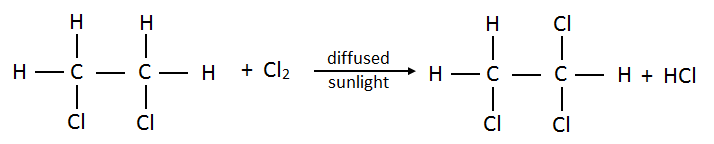
- Dichloroethane:

- Trichloroethane:
- Tetrachloroethane:
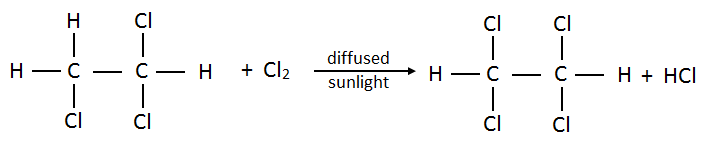
- Pentachloroethane:
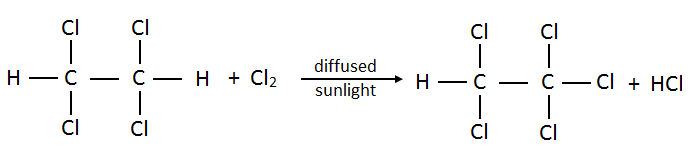
- Hexachloroethane:
17. Differentiate between ethene and ethyne.
|
Ethene |
Ethyne |
|
Ethene have double bond between carbon atoms. |
Ethyne have triple bond between carbon atoms. |
|
Formula: C2H4 |
Formula: C2H2 |
|
Structure:
|
Structure:
|



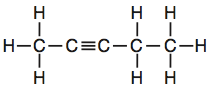




Recent Comments